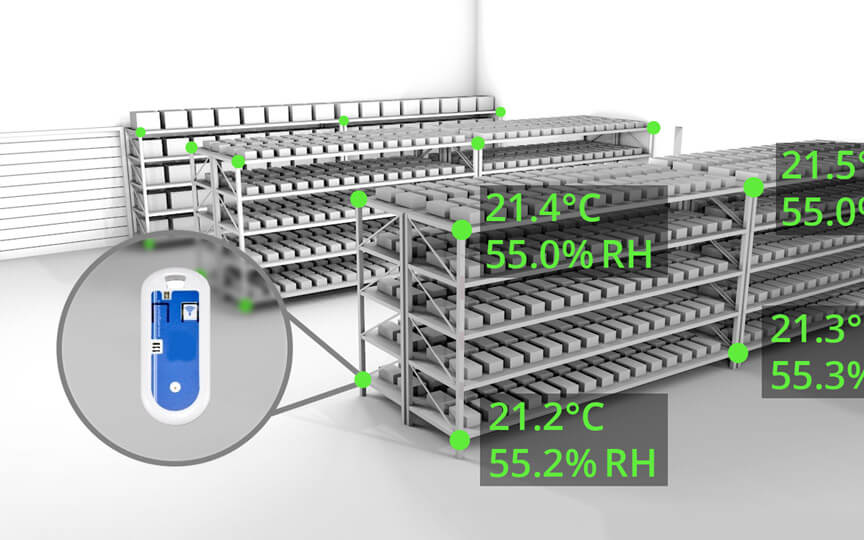
If you consider any space, you will notice that the temperature varies from one location to another. Temperature mapping or Thermal Validation is a concept that is crucial in the pharmaceutical industry.
It records and controls the temperature within a storage area like room, freezer, or warehouse and safeguards the quality of the products.
Let’s see what temperature mapping really means.
Temperature Mapping
It is a procedure for estimating whether a storage area can maintain the necessary temperature.
Temperature mapping utilizes sensors and data loggers which identifies and detects whether a storage area maintains its temperature within its pre-defined limits. The procedure must be able to explain the temperature profile across the storage area under both empty and loaded conditions.
Temperature mapping identifies areas that cannot be utilized for storing temperature-sensitive products near cooling coils, or heat sources.
It is also important that the analysis demonstrates the time required for temperatures to cross the established temperature mapping guidelines in case of power cuts.
Where It Is Used
Temperature mapping is recommended for storage sites under specified conditions prior to usage.
It is important to position the thermal mapping equipment as per the outcomes of the mapping exercise. Similarly, temperature mapping needs to be repeatedly conducted based on the risk assessment results.
In premises composed of a few square meters at room temperature, potential risks need to be assessed according to which, the temperature monitors would be placed. The data loggers should be placed such that the temperatures across horizontal, vertical, and depth plains are recorded along with locations prone to temperature fluctuations.
Some Case Scenario’s
Corporate-level inspections revealed that some companies are unsure of the expectations in terms of compliance with the GDP guidelines.
This lack of clarity can be especially observed in the case of new companies and those having small storage space. Companies with large storage areas can use the expertise of a third-party organization having expertise in the area.
Some temperature mapping guidelines just mention the need for temperature mapping of storage areas without entailing the details of the procedure.
Significance of Temperature Mapping
A mapping exercise of the proposed storage area will ensure that the company will understand its storage area and identify any potential areas therein that may be unsuitable for storing medicines.
The suitability of a controlled temperature chamber for storing temperature-sensitive pharmaceuticals and ambient products can be understood by temperature mapping.
A mapping exercise of a potential storage area must be conducted to ensure that the company is familiar with the necessary compliance and recognizes any unsuitable areas for medicine storage.
When is the Right Time for Temperature Mapping?
It is best to perform temperature mapping during the summer and winter seasons to get an idea of the extreme temperatures and how they may affect the storage area.
The ideal time for a thermal exercise is before storing the stock; however, it may not be feasible if a storage area is being redesigned.
If the storage area is small and without many items already stored, then dummy products can be placed to induce normal operational storage conditions. Temperature mapping can be repeated in such empty storage areas when the stock is fully unloaded.
The seasonal changes must be taken into consideration while repeating the thermal exercise.
The data retrieved from the temperature mapping procedure needs to be documented along with a risk assessment that identifies hot or cold spots, if any.
Conducting Risk Assessment
The first mapping exercise is followed by a risk assessment. In this stage, the data should be recorded, and any associated risks must be assessed.
Risk assessment is carried out since it allows the estimation of suitable positions for permanent monitoring probes.
It would also identify and determine the consistency of future mapping exercises. The process needs to cover all the storage areas with high-temperature variance or those indicating any hot or cold spots.
The exercise can be repeated during seasonal variations or under conditions where the storage area is being reconfigured. Risk assessment should be reviewed periodically and can be integrated into the self-audit process.
Who is a Responsible Person (RP) for Temperature Mapping
A responsible person (RP) is someone who is primarily involved in the mapping process.
The RP needs to be well-acquired with the entire procedure, including findings, risk assessment recommendations, and review process.
The duties of the RP are not restricted to performing temperature mapping. He/she should be capable of illustration supervision and reviewing subsequent minimum/maximum temperature monitoring and recording of data daily.
Also, if any incidents of temperature excursions occur, then the RP is the person of contact.
How does Temperature Mapping Help
Temperature mapping serves as an effective mechanism for maintaining the quality of pharmaceuticals.
It ensures compliance with storage and temperature mapping guidelines.
The storage area must be carefully analyzed for placing the temperature monitoring equipment at the appropriate locations for a successful mapping procedure.
We at Incepbio have understood the requirements of the pharmaceuticals industry and thus are providing thermal validation services that support the critical operations of companies like Biocon, Syngene, Mylan.
To learn more about our Thermal Validation Services click below.